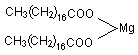1.BP2005/USP31
2.The product is mainly used as troche and the lubricant of the capsule, or anti-viscous agent
|
 CAS: 557-04-0 CAS: 557-04-0
Structure: 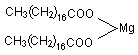  Specifications: Conforms to standard USP27 and BP2000. Specifications: Conforms to standard USP27 and BP2000.
| Text | USP31 | BP2005 | | Assay% | 4.0-5.0(Mg) | 4.5-5.0(Mg) | | Chloride% | ≤0.1 | ≤0.1(0.25%) | | Sulfate% | ≤1.0 | ≤0.5 | | Loss on drying% | ≤6.0 | ≤6.0 | | Iron% | | ≤0.01 | | Lead ppm | ≤10 | ≤10 | | Cadmium | | ≤3ppm | | Acid or Alkalinity | conforms | conforms | | Stearic acid and plam | | | | acid relative density% | ≥90 | ≥90 | | Total assay(stearic acid)% | ≥40 | ≥40 | | Organic Volatile Impurities | conforms | conforms |
 Physical properties: Physical properties:
Character: White, tiny and smooth, fluffy powder;insoluble in water, alcohol, ether; slightly dissoluble in hot alcohol and benzene, and can be decomposed bydilute acid; hard to flow, Viscous powders.
Particle size: 100 percent through 80mesh sieve.  USE: The product is mainly used as troche and the lubricant of the capsule, or anti-viscous agent, the concentration of which is commonly 0.25-2.0 percent.Because of its hydrophobicity, it can delay the effusing rate of the solid preparation medicament.Thus its use concentrations low by all means, and it should be avoided to be used with medicaments which is not match to the alkaline substance. USE: The product is mainly used as troche and the lubricant of the capsule, or anti-viscous agent, the concentration of which is commonly 0.25-2.0 percent.Because of its hydrophobicity, it can delay the effusing rate of the solid preparation medicament.Thus its use concentrations low by all means, and it should be avoided to be used with medicaments which is not match to the alkaline substance.
 Package: It is packed in fiber bag and lining with polyethylene film bag Net Weight 20KG per bag. Package: It is packed in fiber bag and lining with polyethylene film bag Net Weight 20KG per bag.
|
|